0.400 mole of sucrose, 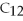
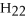
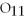 ,contains ________ moles of C.
,contains ________ moles of C.
A) 12
B) 6.02 × 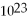
C) 5.02 × 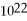
D) 2.89 × 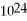
E) 4.80
Correct Answer:
Verified
Q1: What is the molar mass of
Q1: Calculate the molar mass of potassium chloride,KCl.
A)74.55
Q2: One mole of helium gas weighs _.
A)1.000
Q8: 0.100 mole of lithium has a mass
Q8: Avogadro's number is the number of _.
A)particles
Q9: What is the molar mass of sodium
Q10: What is the molar mass of sucrose
Q11: One mole of copper(II)sulfate,CuS Q16: What is the molar mass of copper(II)sulfate,CuSO4 Q19: Calculate the molar mass of magnesium chloride,MgCl2![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents