The molar mass of 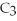
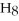
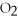 is ________.
is ________.
A) 76.09 g/mole
B) 60.09 g/mole
C) 29.02 g/mole
D) 69.02 g/mole
E) 52.01 g/mole
Correct Answer:
Verified
Q8: Avogadro's number is the number of _.
A)particles
Q9: What is the molar mass of sodium
Q10: What is the molar mass of sucrose
Q11: One mole of copper(II)sulfate,CuS Q12: The molar mass of potassium is _. Q14: How many moles of water, H2O,are present Q15: One mole of particles of any substance Q16: One mole of copper(II)sulfate,CuSO4,contains _ moles of Q17: One mole of neon atoms has a Q18: 0.400 mole of sucrose, C12H22O11,contains _ C![]()
A)19.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents