For the following question(s) ,consider the following balanced equation. 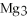
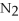 + 6
+ 6 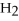 O → 3
O → 3 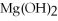 + 2
+ 2 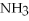
-Find the mass of 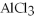 that is produced when 25.0 grams of
that is produced when 25.0 grams of 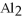
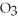 reacts with HCl according to the following equation.
reacts with HCl according to the following equation. 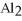
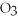 + 6HCl → 2
+ 6HCl → 2 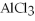 + 3
+ 3 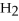 O
O
A) 155 g
B) 72.9 g
C) 65.3 g
D) 32.6 g
E) 16.3 g
Correct Answer:
Verified
Q67: The _ is the minimum energy needed
Q68: A reaction that releases energy as it
Q71: Any reaction that absorbs 150 kcal of
Q73: Barium chloride and sodium sulfate react according
Q75: Answer the question(s)below about the following reaction.
2
Q78: Answer the question(s)below about the following reaction.
2
Q78: For the following question(s),consider the following balanced
Q79: What type of reaction is: 
Q81: When 25.0 g of KCl 
Q82: When the following equation is balanced,the coefficient
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents