For the following question(s) ,consider the following balanced equation. 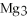
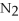 + 6
+ 6 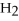 O → 3
O → 3 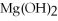 + 2
+ 2 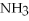
-How many grams of NO are required to produce 145 g of 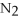 in the following reaction?
in the following reaction?
4 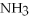 + 6NO → 5
+ 6NO → 5 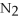 + 6
+ 6 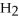 O
O
A) 186 g
B) 155 g
C) 125 g
D) 129 g
E) 145 g
Correct Answer:
Verified
Q53: For the following question(s),consider the following equation.
2Mg
Q60: For the following question(s),consider the following balanced
Q63: Answer the question(s)below about the following reaction.
2
Q65: For the following question(s),consider the following balanced
Q66: For the following question(s),consider the following balanced
Q67: If the reaction shown below is exothermic,the
Q70: Barium chloride and sodium sulfate react according
Q74: Barium chloride and sodium sulfate react according
Q79: The _ is the energy difference between
Q92: Answer the question(s)below about the following reaction.
2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents