Answer the question(s) below about the following reaction.
2 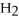
 → 2
→ 2 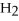 O +
O + 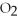
-How many moles of hydrogen peroxide (H2O2) are needed to produce 5.0 moles of water?
A) 1.0 mole
B) 2.0 moles
C) 2.5 moles
D) 4.0 moles
E) 5.0 moles
Correct Answer:
Verified
Q68: A reaction that releases energy as it
Q81: When 25.0 g of KCl 
Q82: When the following equation is balanced,the coefficient
Q86: What type of reaction is the following?
Zn
Q87: The number of particles in 1 mole
Q89: The molar mass of magnesium is 24.31
Q89: The following reaction is correctly balanced.
KCl
Q91: What type of reaction is the following?
2KClO3
Q102: The molar mass of chlorine gas is
Q108: The molar mass of copper(II)chloride is 134.5
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents