The molar mass of copper(II)nitrate,Cu(N 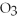
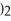 is ________.
is ________.
Correct Answer:
Verified
Q93: Avogadro's number is the number of grams
Q94: What is the mass,in grams,of 1.00 mole
Q95: The mass of one mole of water
Q97: Which reactant is oxidized in the following
Q99: When the following equation is balanced,the coefficient
Q101: The following is a double replacement reaction.
2
Q102: The following is a decomposition reaction.
2KClO3 →
Q104: In the following reaction Al is oxidized.
2Al
Q105: In the following reaction,20.0 g of Al
Q106: In the reaction of hydrogen gas with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents