A balloon is filled with helium gas.For the following question(s) ,select the letter of the balloon diagram that corresponds to the given change in conditions. 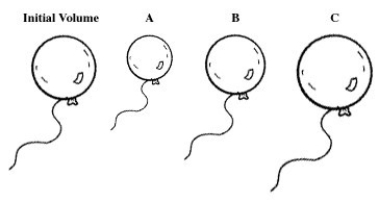
-The temperature is changed from 50 °C to -150 °C at constant pressure.
A) A
B) B
C) C
D) A and B
E) B and C
Correct Answer:
Verified
Q15: Which of the following correctly describes the
Q16: In response to Boyle's law,the pressure of
Q17: Which of the following is not part
Q18: A balloon is filled with helium gas.For
Q19: Which measurement describes the pressure of a
Q21: At STP,what is the volume of 4.50
Q22: A sample of argon at 300.°C and
Q23: How many moles of neon occupy a
Q24: In Gay-Lussac's law,the pressure of a gas
Q25: At STP conditions,11 g of SO2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents