A gas sample containing 12 g of 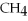 has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)
has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)
A) 22.4.L
B) 53 L
C) 56 L
D) 70 L
E) 3.5 L
Correct Answer:
Verified
Q24: In Gay-Lussac's law,the pressure of a gas
Q28: Which of the following correctly describes the
Q30: A sample of nitrogen gas had a
Q31: At 570.mmHg and 25 °C,a gas sample
Q32: A gas sample contains 4.0 g of
Q33: A gas at 5.00 atm pressure was
Q34: A tank contains helium gas at 490
Q35: At STP,temperature and pressure have the values
Q52: The total pressure in a mixture of
Q58: A cyclopropane-oxygen mixture is used as an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents