A tank contains helium gas at 490 mmHg,nitrogen gas at 0.75 atm,and neon at 520 torr.What is the total pressure in atm?
A) 2.1 atm
B) 0.55 atm
C) 1.0 × 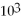 atm
atm
D) 1.5 atm
E) 1600 atm
Correct Answer:
Verified
Q29: A gas sample containing 12 g of
Q30: A sample of nitrogen gas had a
Q31: At 570.mmHg and 25 °C,a gas sample
Q32: A gas sample contains 4.0 g of
Q33: A gas at 5.00 atm pressure was
Q35: A gas contained in a steel tank
Q37: A sample of argon at 300.°C and
Q38: At STP conditions,11 g of 
Q39: According to Avogadro's law,_.
A)the volume of a
Q39: A diver exhales a bubble with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents