Matching
Indicate whether each of the following compounds dissolves in water to give ions,molecules,or both.
Premises:
HF,a weak electrolyte
urea,a nonelectrolyte
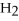
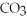 ,a weak electrolyte
,a weak electrolyte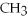
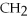 OH,a nonelectrolyte
OH,a nonelectrolyteNaCl,a strong electrolyte
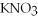 ,a soluble salt
,a soluble saltglucose,a nonelectrolyte
Responses:
both
ions
molecules
Correct Answer:
Premises:
Responses:
HF,a weak electrolyte
urea,a nonelectrolyte
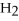
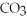 ,a weak electrolyte
,a weak electrolyte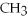
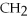 OH,a nonelectrolyte
OH,a nonelectrolyteNaCl,a strong electrolyte
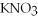 ,a soluble salt
,a soluble saltglucose,a nonelectrolyte
Premises:
HF,a weak electrolyte
urea,a nonelectrolyte
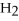
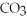 ,a weak electrolyte
,a weak electrolyte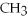
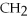 OH,a nonelectrolyte
OH,a nonelectrolyteNaCl,a strong electrolyte
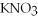 ,a soluble salt
,a soluble saltglucose,a nonelectrolyte
Responses:
Related Questions
Q61: A solution which has 12 g of
Q63: If a red blood cell is placed
Q68: Which has a higher osmotic pressure 1.0
Q69: A 2.0% (m/v)NaCl solution contains _ g
Q70: If 450.mL of 6.0 M KBr solution
Q70: When KCl is added to water,the salt
Q71: If a red blood cell is placed
Q72: 200.mL of a 12.0% (m/v)NaCl solution is
Q72: Match the type of mixture with the
Q73: When 50.mL of 10.% (m/v)NaCl is diluted
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents