When KCl dissolves in water ________.
A) the 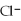 ions are attracted to dissolved
ions are attracted to dissolved 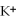 ions
ions
B) the 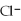 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
C) the 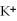 ions are attracted to
ions are attracted to 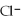 ions on the KCl crystal
ions on the KCl crystal
D) the 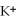 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
E) the 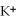 ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
Correct Answer:
Verified
Q1: According to Henry's law,the solubility of a
Q8: Oil does not dissolve in water because
Q9: A mixture is prepared by dissolving 2
Q12: A solution contains 43 mEq/L of of
Q12: When some of the sugar added to
Q13: In a solution,the solvent _.
A)is a liquid
B)can
Q14: Water is a polar solvent and hexane
Q17: How many equivalents are in 0.60 mole
Q18: Which of the following molecules can form
Q19: An increase in the temperature of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents