Matching
Identify the following as acids, bases, or neutral solutions.
Premises:
has a sour taste
pH =9.0
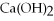
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M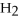 O
Oturns blue litmus paper red
Responses:
base
acid
neutral
Correct Answer:
Premises:
Responses:
has a sour taste
pH =9.0
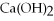
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M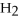 O
Oturns blue litmus paper red
Premises:
has a sour taste
pH =9.0
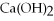
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M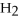 O
Oturns blue litmus paper red
Responses:
Related Questions
Q42: HBr is a strong acid.
Q53: A system is at equilibrium when the
Q58: The name of H2SO4 is hydrosulfuric acid.
Q59: Strong acids react with Zn metal to
Q61: In the following solutions,is the [OH-] greater
Q62: A buffer is a solution that tends
Q64: Identify each of the following compounds as
Q65: If the carbon dioxide level in the
Q66: For many reactions of acids with bases,the
Q67: Alkalosis is the blood condition in which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents