Consider the following specific heats of metals. 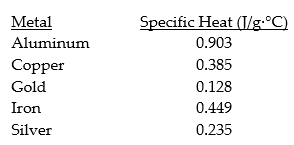
If the same amount of heat is added to 50.0 g samples of each of the metals,which are all at the same temperature,which metal will reach the highest temperature?
A) aluminum
B) copper
C) gold
D) iron
E) silver
Correct Answer:
Verified
Q96: How many kilojoules are there in 95.0
Q97: An energy diagram that shows the reactants
Q98: Melting point can be defined as the
Q99: If a particular process is endothermic,the reverse
Q100: What is the value of 335 K
Q102: What is the value of 23°C on
Q103: How many grams of water when supplied
Q104: What is the value of -25°C on
Q105: What is the value of 783 K
Q106: The temperature of 390 kelvin is warmer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents