Suppose it took 108 joules of energy to raise a bar of gold from 25.0°C to 29.7°C.Given that the specific heat capacity of gold is 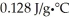 ,what is the mass (in grams) of the bar of gold?
,what is the mass (in grams) of the bar of gold?
A) 6.5 × 101 g
B) 1.8 × 102 g
C) 1.28 × 102 g
D) 1.08 × 102 g
E) none of the above
Correct Answer:
Verified
Q105: What is the value of 783 K
Q106: The temperature of 390 kelvin is warmer
Q107: How many joules are in 55.2 calories?
A)13,200
B)55,200
C)13.2
D)231
E)none
Q108: What is the specific heat (J/g∙°C)of a
Q109: A 15.0 gram lead ball at 25.0°C
Q111: When 49.5 J of heat was transferred
Q112: Given the table of specific heat values
Q113: How many Calories are in 575.0 calories?
A)575,000
B)0.5750
C)137.6
D)2,404
E)none
Q114: Given the table of specific heat values
Q115: What is the final temperature of 25.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents