If 3.011 ×1023 molecules have a mass of 20.04 grams,what is the molar mass of this substance?
A) 40.08 g/mol
B) 10.02 g/mol
C) 20.04 g/mol
D) 6.658 × 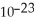 g/mol
g/mol
E) none of the above
Correct Answer:
Verified
Q60: How many hydrogen atoms are in 35.0
Q61: How many molecules of sulfur trioxide are
Q62: What is the molar mass of aluminum
Q63: A 500.gram iron ore sample was determined
Q64: A 42.7 gram sample of potassium nitrate
Q66: One mole of potassium sulfate contains:
A)4 moles
Q67: Calculate the molar mass of calcium nitrate.
A)136.03
Q68: Which of the following contains 9.02 ×
Q69: The mass of one mole of carbon
Q70: One mole of (NH4)2HPO4 contains how many
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents