What is the molecular formula of a compound given the molar mass of the compound is 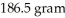 and the empirical formula is C2H7?
and the empirical formula is C2H7?
A) C4H14
B) C3H21
C) C2H7
D) C2H14
E) none of the above
Correct Answer:
Verified
Q89: The empirical formula of a compound:
A)describes the
Q90: The chemical formula CH2O can be classified
Q91: What is the mass percent of hydrogen
Q92: Which of the following is already in
Q93: The simplest formula for hydrogen peroxide is
Q95: An estrogen compound with the empirical formula
Q96: Vitamin C is known chemically by the
Q97: A 7.96 gram sample of silver reacts
Q98: A chromium oxide compound contains 104.0 grams
Q99: Determine the empirical formula of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents