If you have 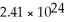 atoms of copper,how many moles of copper do you have?
atoms of copper,how many moles of copper do you have?
A) 0.250
B) 4.00
C) 0.600
D) 3.00
E) none of the above
Correct Answer:
Verified
Q98: A chromium oxide compound contains 104.0 grams
Q99: Determine the empirical formula of a compound
Q100: A compound composed of only carbon and
Q101: What is the molecular formula of a
Q102: A compound has a molar mass of
Q104: An unknown acid has a molar mass
Q105: What is the value of n when
Q106: What would the empirical formula be for
Q107: How many atoms are present in 4.5
Q108: Given that sodium chloride is 39.0% sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents