If the theoretical yield of the reaction below corresponds to 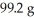 and the actual yield was 60.9 g,calculate the percent yield.
and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
A) 16.0 %
B) 71.8 %
C) 38.0 %
D) 61.4 %
E) none of the above
Correct Answer:
Verified
Q100: What is the excess reactant for the
Q101: Consider the reaction: 2 Al + 3Br2
Q102: How many grams of water are needed
Q103: Suppose a computer manufacturer can build circuit
Q104: Consider the following equation: CO + 2
Q106: How many grams of the excess reactant
Q107: How many moles of water are needed
Q108: What is the percent yield of CuS
Q109: If the theoretical yield of the reaction
Q110: Hydrochloric acid reacts with barium hydroxide according
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents