The possible values for the principal quantum numbers are: 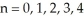 .
.
Correct Answer:
Verified
Q28: A principal shell with a value of
Q29: The higher the principal quantum number,the lower
Q30: The orbital diagram for fluorine shows 1
Q31: The ground state is when an electron
Q32: The subshells of the orbital are represented
Q34: Electrons behave like particles and we can
Q35: The energy of an electron orbit is
Q36: The correct electron configuration for magnesium is:
Q37: An emission spectrum results when light emitted
Q38: The energy of each Bohr orbit increases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents