The correct Lewis structure for 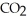 shows that the molecule contains two double bonds.
shows that the molecule contains two double bonds.
Correct Answer:
Verified
Q12: The triple bond present in diatomic nitrogen,N2,is
Q13: A chemical bond is classified as a
Q14: Lewis theory predicts that the formula for
Q15: Having eight valence electrons is very stable
Q16: The Lewis theory predicts that the formula
Q18: Drugs to fight HIV have been developed
Q19: Chlorine has 8 valence electrons in the
Q20: The Lewis structure of water has two
Q21: Which Lewis structure below correctly represents the
Q22: When you have 4 electron groups and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents