The Lewis structure for carbon monoxide is 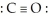 This structures shows:
This structures shows:
A) 4 lone pairs and 1 bonding pair.
B) 4 lone pairs and 3 bonding pairs.
C) 2 lone pairs and 3 bonding pairs.
D) 2 lone pairs and 1 bonding pair.
E) none of the above
Correct Answer:
Verified
Q36: Carbon monoxide contains resonance Lewis structures.
Q37: The electron geometry of a molecule is
Q38: Boron forms compounds that violate the octet
Q39: The correct Lewis structure for CO2 shows
Q40: Resonance structures are the best representation we
Q42: The total number of electrons to be
Q43: The Lewis model predicts that the formula
Q44: The central atom in the chlorate anion,ClO3-
Q45: What is the correct Lewis structure for
Q46: What is the correct Lewis structure for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents