Which set shows the correct resonance structures for SeO2?
A) : 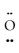 -
- 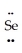 -
- 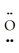 : ↔
: ↔ 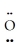 =
= 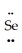 =
= 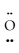
B) : 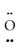 =
= 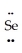 -
- 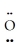 : ↔ :
: ↔ : 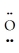 -
- 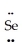 =
= 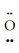 :
:
C) : 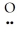 =Se=
=Se= 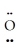 ↔ : O≡ Se-
↔ : O≡ Se- 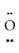 :↔ :
:↔ : 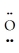 -Se≡ O :
-Se≡ O :
D) 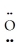 =
= 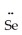 -
- 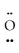 : ↔ :
: ↔ : 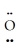 -
- 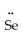 =
= 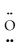
E) SeO2 does not have a resonance structure.
Correct Answer:
Verified
Q58: The Lewis model predicts that the formula
Q59: How many bonding electrons are in the
Q60: What is the correct Lewis structure for
Q61: What is the molecular geometry if you
Q62: What is the angle between electron groups
Q64: What is the electron geometry if you
Q65: What is the molecular geometry if you
Q66: Which molecule below would have a Lewis
Q67: The correct Lewis structure for BF3 would
Q68: What is the molecular geometry of ozone,O3?
A)bent
B)linear
C)tetrahedral
D)trigonal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents