The initial volume of a gas cylinder is 750.0 mL.If the pressure of a gas inside the cylinder changes from 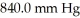 to
to 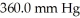 ,what is the final volume the gas occupies?
,what is the final volume the gas occupies?
A) 3.151 L
B) 630.0 mL
C) 1.750 L
D) 321.4 mL
E) none of the above
Correct Answer:
Verified
Q47: STP conditions are 273 K and 760
Q48: All of the following statements are consistent
Q49: Boyle's Law is expressed as:
A)V is proportional
Q50: What is the equivalent pressure of 760
Q51: Divers often inflate heavy duty balloons attached
Q53: The volume of 9.00 grams of water
Q54: A 22.4 liter sample of gas at
Q55: A balloon filled with 0.500 L of
Q56: 1 torr is equal to:
A)760 mm Hg.
B)1
Q57: To solve problems using Boyle's Law,which mathematical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents