A sample of helium gas initially at 37.0°C,785 torr and 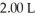 was heated to 58.0°C while the volume expanded to
was heated to 58.0°C while the volume expanded to 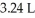 .What is the final pressure in atm?
.What is the final pressure in atm?
A) 517
B) 0.681
C) 1.79
D) 3.21
E) none of the above
Correct Answer:
Verified
Q72: Which of the following statements is TRUE
Q73: Charles's Law is expressed as:
A)V is proportional
Q74: What is the final volume of a
Q75: Which one of the following is impossible
Q76: If the volume of a gas container
Q78: What is the initial temperature (°C)of a
Q79: A gas sample occupies 3.50 liters of
Q80: A certain volume of gas was confined
Q81: What is the major component of the
Q82: What is the temperature (°C)of 2.48 moles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents