How many moles of gas were added to a balloon that started with 2.3 moles of gas and a volume of 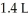 given that the final volume was
given that the final volume was 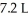 ?
?
A) 9.5
B) 4.4
C) 12
D) 0.085
E) none of the above
Correct Answer:
Verified
Q90: The ideal gas law is:
A)PV = nRT
B)P
Q91: Which conditions can cause nonideal gas behavior
Q92: If each of the following gas samples
Q93: If a mixture of gases contained 78%
Q94: Avogadro's Law is expressed as:
A)V is proportional
Q96: Which of the following gas law relationships
Q97: What problem could happen if deep sea
Q98: What is the third most abundant component
Q99: To solve problems using Avogadro's Law,which mathematical
Q100: A gas cylinder contains only the gases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents