What is the pressure of a 3.00 L gas vessel that has 18.0 grams of helium at 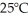 ? (R= 0.0821 L atm/ mol K)
? (R= 0.0821 L atm/ mol K)
A) 147 atm
B) 36.7 atm
C) 32.6 atm
D) 1.81 atm
E) none of the above
Correct Answer:
Verified
Q82: What is the temperature (°C)of 2.48 moles
Q83: A "shielding gas" mixture of argon and
Q84: A 3.76 g sample of a noble
Q85: A sample of 0.255 mole of gas
Q86: For an ideal gas,which of the following
Q88: Which of the following diatomic elements would
Q89: What happens to the volume of a
Q90: The ideal gas law is:
A)PV = nRT
B)P
Q91: Which conditions can cause nonideal gas behavior
Q92: If each of the following gas samples
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents