Which of the following diatomic elements would have a mass of 19.08 grams stored in a 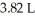 container at
container at 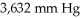 and 100°C? (R= 0.0821 L atm/ mol K)
and 100°C? (R= 0.0821 L atm/ mol K)
A) 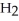
B) 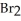
C) 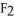
D) 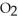
E) not enough information.
Correct Answer:
Verified
Q83: A "shielding gas" mixture of argon and
Q84: A 3.76 g sample of a noble
Q85: A sample of 0.255 mole of gas
Q86: For an ideal gas,which of the following
Q87: What is the pressure of a 3.00
Q89: What happens to the volume of a
Q90: The ideal gas law is:
A)PV = nRT
B)P
Q91: Which conditions can cause nonideal gas behavior
Q92: If each of the following gas samples
Q93: If a mixture of gases contained 78%
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents