How much energy does it take to melt a 16.87 g ice cube? 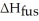 = 6.02 kJ/mol
= 6.02 kJ/mol
A) 102 kJ
B) 108 kJ
C) 936 J
D) 5.64 kJ
E) none of the above
Correct Answer:
Verified
Q56: Which statement about boiling point is FALSE?
A)The
Q57: Water has unusual physical properties which enable
Q58: Which of the following statements is false?
A)Evaporation
Q59: Which state of matter has a high
Q60: Intermolecular forces are responsible for:
A)the taste sensations.
B)the
Q62: The ability of sodium chloride to mix
Q63: What is the heat of vaporization(kJ/mol)if it
Q64: The amount of heat required to melt
Q65: How many kilojoules of heat are needed
Q66: Gaseous water vapor can frost the windows
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents