Multiple Choice
What is the pH of a solution that has a H⁺ concentration equal to 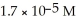 ?
?
A) 4.77
B) 5.20
C) 0.22
D) 10.20
E) none of the above
Correct Answer:
Verified
Related Questions
Q87: Which solution below has the highest concentration
Q88: The pH of a solution is 5.00.Which
Q89: A solution at 25°C has a hydrogen
Q90: Substances that can act both as an
Q91: What is the value of the ion
Q93: Consider a 1.0 X 10-7 M HCl
Q94: What is the concentration of H⁺ in
Q95: What is the concentration of the hydronium
Q96: In order for a solution to be
Q97: If the pH of an aqueous solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents