For the reaction 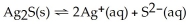 ,
, 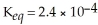 and the equilibrium concentration of silver ion is
and the equilibrium concentration of silver ion is 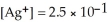 M.What is
M.What is 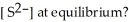
A) 0.0038
B) 9.6 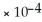
C) 2.6 × 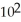
D) 1.0 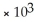
E) none of the above
Correct Answer:
Verified
Q66: Which equilibrium constant represents a reaction that
Q67: For the reaction 2 H2O (l)⇌ 2H2
Q68: For the reaction Q69: For the reaction LiOH (s)⇌ Li+ (aq)+ Q70: Which equilibrium constant represents a reaction that Q72: For the reaction 2 A ⇌ B,the Q73: Le Chatelier's Principle states that: Q74: For the reaction Q75: What must be TRUE for a reaction Q76: Which of the following is TRUE of![]()
A)a disturbing force![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents