Given the choice of adding ions of Mg 2+ (aq) ,Ca 2+ (aq) ,Sr 2+ (aq) or Ba 2+ (aq) to a solution of carbonate ions,CO3 2-,which choice would precipitate an insoluble carbonate compound first? Use the following  data:
data: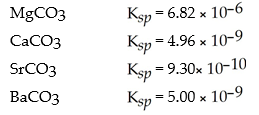
A) Mg 2+
B) Ca 2+
C) Sr 2+
D) Ba 2+
E) none of these will give a precipitate
Correct Answer:
Verified
Q88: Consider the following endothermic equilibrium reaction:
Fe2O3
Q89: For the reaction Q90: For the reaction Q91: For the reaction H2 (g)+ Cl2 (g)⇌ Q92: For the reaction Q94: For the reaction Q95: Consider the reaction: Q96: What is the molar solubility,(S),of FeS if Q97: What happens to the equilibrium position of Q98: Consider the reaction: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()
![]()

