Multiple Choice
Calculate the equilibrium constant (Keq) for the reaction 1A (aq) + 1B (aq) ⇌ 2C (aq)
given that 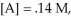
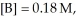 and
and 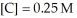 at equilibrium.
at equilibrium.
A) 9.9
B) 0.40
C) 2.5
D) 20.
E) none of the above
Correct Answer:
Verified
Related Questions
Q105: What is the [ Q106: What is the [ Q107: Which statement below is FALSE? Q108: The effect of a catalyst is to: Q109: What is the molar solubility,(S),of FeCO3 if Q111: What is the value for the Q112: Which statement about activation energy is FALSE? Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
A)The rate of
A)increase
A)Activation

