Determine
(i) the oxidation number of the metal,
(ii) the number of d electrons,
(iii) the coordination number,
(iv) the charge of the complex ion, and
(v) the number and type of ligands for the coordination compound shown below. 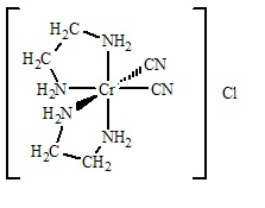
A) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = zero monodentate and four bidentate
B) i = +3; ii = 2 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
C) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = two monodentate and one bidentate
D) i = +3; ii = 3 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
E) None of the above have all five answers correctly presented
Correct Answer:
Verified
Q79: What is the oxidation number of Fe
Q80: Ethylenediaminetetraacetic acid (EDTA) is
A) not useful as
Q81: The neutral monodentate ligand L forms the
Q82: Predict the number of unpaired electrons in
Q83: In transition metal complexes, the metal ions
Q85: Predict the number of unpaired electrons in
Q86: The correct name of the complex ion
Q87: The oxidation number of Co in [Co(NH3)4Cl2]Cl
Q88: Predict the number of unpaired electrons in
Q89: Bidentate and polydentate ligands are also called
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents