Nitric acid is formed by the gas-phase hydrolysis of N2O5. The energy profile curve for the reaction N2O5 + H2O 2HNO3 is shown here. The reaction is endothermic and the activation energy of the reverse reaction is larger than for the forward reaction. 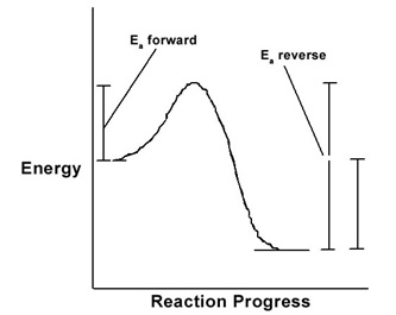
Correct Answer:
Verified
Q96: For the reaction whose rate law is
Q97: Which of the following statements is false
A)
Q98: For the reaction X2 + Y
Q99: The peroxodisulfate ion can oxidize iodide
Q100: Nitrous oxide (N2O) decomposes at 600
Q102: At a certain temperature, the data
Q103: The rate constant for a certain first-order
Q104: Use the table of data shown
Q105: Given the rate law for a reaction,
Q106: For the first-order reaction 2N2O5
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents