N,N-diethyl-m-tolumide (DEET) is the active ingredient in many mosquito repellents. What is the hybridization state of the nitrogen atom in the structure of DEET shown below 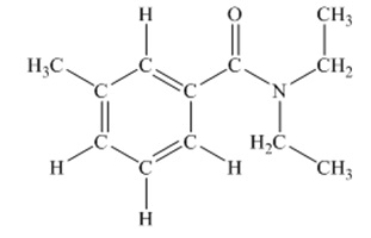
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:
Verified
Q65: According to Valence Bond Theory, which orbital
Q66: What is the hybridization of the As
Q67: Indicate the type of hybrid orbitals used
Q68: Indicate the type of hybrid orbitals used
Q69: Ibuprofen is used as an analgesic for
Q71: Indicate the type of hybrid orbitals used
Q72: The hybridization of the central nitrogen atom
Q73: N,N-diethyl-m-tolumide (DEET) is the active ingredient in
Q74: N,N-diethyl-m-tolumide (DEET) is the active ingredient in
Q75: Indicate the type of hybrid orbitals used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents