The number of pi bonds in the molecule below is 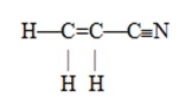
A) 1
B) 2
C) 3
D) 5
E) 9
Correct Answer:
Verified
Q92: According to Molecular Orbital Theory, two
Q93: According to Molecular Orbital Theory, two
Q94: Consider the species N2-, N2, and N2+.
Q95: What is the bond order of Cl2+
A)
Q96: In which of the following would the
Q98: Consider the species Cl2+, Cl2, and Cl2-.
Q99: A sp2 hybridized central boron atom
Q100: A sp hybridized terminal nitrogen atom
Q101: In VSEPR theory the molecular geometry of
Q102: The electrons in the delocalized molecular orbitals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents