Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 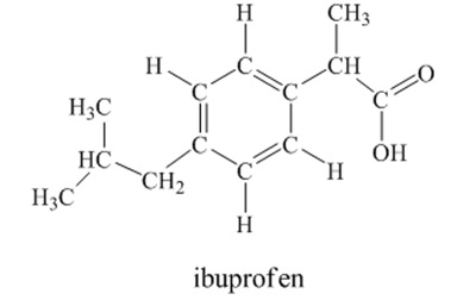 How many sigma bonds and pi bonds are contained in an ibuprofen molecule
How many sigma bonds and pi bonds are contained in an ibuprofen molecule
33 sigma bonds and 4 pi bonds
Correct Answer:
Verified
Q95: What is the bond order of Cl2+
A)
Q106: Which of the following correctly lists species
Q107: Which of the following molecules has polar
Q108: In VSEPR theory the molecular geometry of
Q109: CO2 is nonpolar, but OCS is polar.
Q110: N,N-diethyl-m-tolumide (DEET) is the active ingredient in
Q112: According to VSEPR theory, which of the
Q114: Which of the following correctly lists species
Q115: Which of the following molecules has polar
Q116: Using periodic trends, arrange the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents