True/False
The following correctly depicts the Lewis dot symbol for the sulfide ion. 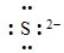
Correct Answer:
Verified
Related Questions
Q96: Which response includes all the molecules below
Q97: Which one of the following molecules has
Q98: What is the formal charge on the
Q99: In the Lewis structure of the iodate
Q100: Estimate the enthalpy change for the
Q102: Calculate the energy required for the
Q103: Use the Born-Haber cycle to calculate
Q104: Covalent bonding is the only type of
Q105: A Lewis structure for SO3 that obeys
Q106: Shown here is a Lewis structure for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents