Carbonic acid, H2CO3, is a weak acid that contributes to the taste and produces the carbon dioxide bubbles in all carbonated beverages. How many valence electrons are used to show the Lewis structure for H2CO3 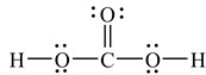
A) 24
B) 22
C) 20
D) 18
E) None of the above
Correct Answer:
Verified
Q107: The structure below depicts the correct Lewis
Q108: The following reaction correctly shows the
Q109: The following Lewis dot symbol correctly depicts
Q110: The following Lewis structure for OF2 uses
Q111: The molecule NH2NH2 shows both ionic and
Q113: The compound Al(ClO3)3 shows only ionic bonding.
Q114: Use bond energies to estimate the enthalpy
Q115: The standard enthalpy of formation of
Q116: Use the bond enthalpy data given
Q117: Use the bond enthalpy data given
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents