Shown here is a Lewis structure for the chlorite ion, ClO2-, that obeys the octet rule, showing all non-zero formal charges. How many resonance structures for ClO2- that obey the octet are possible ?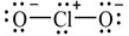
A) four
B) three
C) two
D) one
E) none of the above
Correct Answer:
Verified
Q121: Shown here is the Lewis structure for
Q122: The Lewis structure shown here is correct
Q123: The following Lewis structure depicts the product
Q124: Shown here is a Lewis structure for
Q125: Examine this Lewis structure for the phosphate
Q127: Of the following substances, KCl, KBr, and
Q128: The bond in F2 is described as
Q129: How many resonance phosphate Lewis structures can
Q130: Shown here is a Lewis structure for
Q131: The polarity of covalent bonds increases as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents