Which of the molecules below undergo extensive hydrogen bonding? 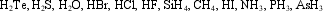
A) HBr, HCl, HF, H2O
B) H2O, HF, NH3
C) CH4, H2O, HF, NH3
D) H2S, H2O, HCl, HF
E) AsH3, NH3, HF, H2S
Correct Answer:
Verified
Q39: How many atoms are bonded to the
Q40: Determine the hybridization around each central atom.
Q41: Which substance experiences dipole-dipole interactions between its
Q42: Which interaction(s) exist(s) between CO molecules?
Q43: Because of London forces, molecules with _
Q45: Which compound experiences hydrogen bonding as one
Q46: What is the major type of force
Q47: Which interaction(s) exist(s) between ClF3 molecules?
I.
Q48: Characterize the polarity of these molecules, in
Q49: Which compound has the lowest boiling point?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents