What is oxidized in the reaction below? 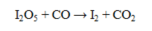
A) CO
B) CO2
C) I2
D) I2O5
E) CO and CO2
Correct Answer:
Verified
Q29: Which of the following represents oxidation?
A) 2
Q30: What is the oxidation number of N
Q31: Which substance is reduced in the reaction
Q32: Which of the following represents reduction?
A) Al
Q33: What is the molarity of a solution
Q35: What is the oxidation number of P
Q36: If a 45.0 mL sample of 2.20
Q37: What is the oxidation number of N
Q38: Which substance is oxidized in the reaction
Q39: Determine the ammonium ion concentration of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents