Oxalic acid, H2C2O4, reacts with Y(NO3) 3 as shown by the equation below. What weight of yttrium oxalate is produced from 50.0 mL of 0.265 M Y(NO3) 3 and excess oxalic acid? Assume that all of the yttrium oxalate is insoluble. 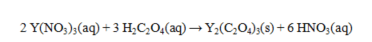
A) 1.46 g
B) 1.82 g
C) 2.93 g
D) 3.64 g
E) 5.85 g
Correct Answer:
Verified
Q36: If a 45.0 mL sample of 2.20
Q37: What is the oxidation number of N
Q38: Which substance is oxidized in the reaction
Q39: Determine the ammonium ion concentration of a
Q40: Which element is least reactive?
A) Al
B) Cu
C)
Q42: Give an example of a strong base.
Q43: When an element is _, it loses
Q44: How many grams of PbI2 will precipitate
Q45: Which of the methods described below will
Q46: Determine the mass of BaSO4 that is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents