How many moles of H2O are formed from the complete combustion of 45.0 g of methanol, CH3OH? 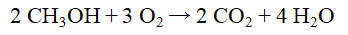
A) 0.711 mol
B) 1.41 mol
C) 1.42 mol
D) 2.81 mol
E) 50.6 mol
Correct Answer:
Verified
Q20: Classify the following reaction. Q21: How many grams of Na2O are formed Q22: The Roman numerals in the reaction given Q23: The Roman numerals in the reaction given Q24: The Roman numerals in the reaction given Q26: How many moles of O2 will be Q27: How many grams of TiCl4 are needed Q28: How many mol of ammonia will be Q29: The Roman numerals in the reaction given Q30: The Roman numerals in the reaction given![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents