In the reaction shown below, ____ is the oxidizing agent and ____ the reducing agent. 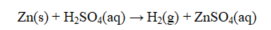
A) Zn2+; H2
B) Zn; H+
C) H2; Zn2+
D) H+; Zn2+
E) H+; Zn
Correct Answer:
Verified
Q10: If cadmium metal and the Fe(III) ion
Q11: Which statement is correct?
A) all electrolytic cells
Q12: Which compound contains the atom with the
Q13: Which is not true of the standard
Q14: Which change describes an oxidation half-reaction?
A) decrease
Q16: The study of the relationships between electron
Q17: Which of these components must be present
Q18: A voltaic cell is one in which
A)
Q19: Exhibit 18-2 Use this list of
Q20: When the reaction shown below is balanced,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents