Exhibit 18-2 Use this list of half-reactions to answer the following question(s) .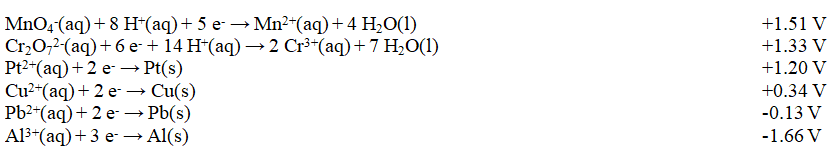 Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
A) Al3+(aq) and Cr3+(aq)
B) Al3+(aq) and Cu(s)
C) Cr2O72-(aq) and MnO4-(aq)
D) Cu(s) and MnO4-(aq)
E) Pt(s) and Pb2+(aq)
Correct Answer:
Verified
Q32: Exhibit 18-2 Use this list of half-reactions
Q33: Which statement concerning the proton exchange membrane
Q34: Which of the following would require the
Q35: A fuel cell is
A) an electrolytic cell
Q36: The relationship between Gibbs free energy
Q38: The value of Ecell at 25
Q39: A secondary cell is one which _;
Q40: A primary battery is one in which
A)
Q41: Is the part of a flashlight battery
Q42: Explain why galvanizing steel chain-link fencing is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents