Consider the cell reaction 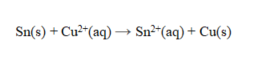 The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
A) -86.3 kJ; 1.26 × 1015
B) -43.1 kJ; 1.37 × 1043
C) 43.1 kJ; 3.55 × 107
D) 86.3 kJ; 7.92 × 10-16
E) 86.3 kJ; 2.00 × 1086
Correct Answer:
Verified
Q24: The value of E
Q25: An electrolytic reaction is a system
Q26: Calculate the value of Ecell for
Q27: Exhibit 18-2 Use this list of half-reactions
Q28: A mass of 0.839 g of a
Q30: Which process could not be an electrolytic
Q31: Which statement about lead storage batteries
Q32: Exhibit 18-2 Use this list of half-reactions
Q33: Which statement concerning the proton exchange membrane
Q34: Which of the following would require the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents