The value of E cell for the cell shown below is + 1.41 V. 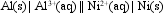
What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq) , 2.0 M?
A) +1.34 V
B) +1.38 V
C) +1.41 V
D) +1.44 V
E) +1.48 V
Correct Answer:
Verified
Q16: The study of the relationships between electron
Q17: Which of these components must be present
Q18: A voltaic cell is one in which
A)
Q19: Exhibit 18-2 Use this list of
Q20: When the reaction shown below is balanced,
Q22: The value of E
Q23: Exhibit 18-2 Use this list of half-reactions
Q24: The value of E
Q25: An electrolytic reaction is a system
Q26: Calculate the value of Ecell for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents