The conjugate base of NH3 is ____.
A) H2O
B) OH-
C) 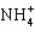
D) NH4OH
E) 
Correct Answer:
Verified
Q2: In terms of acid strength, which acid
Q3: Which reaction illustrates water acting as a
Q4: A Bronsted-Lowry base must have a(n) _.
A)
Q5: A Bronsted-Lowry acid is always
A) a highly
Q6: Which cannot act as a Bronsted-Lowry acid?
A)
Q8: Bronsted-Lowry acids _ and Bronsted-Lowry bases _.
A)
Q9: Which pair of substances represents a conjugate
Q10: Because water can act as a Bronsted-Lowry
Q11: Amines have the general formula
A) R-CHO
B) CH3-(CH2)n-CH3
C)
Q12: In this reaction ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents