The freezing points of the following aqueous solutions, from highest to lowest, are: 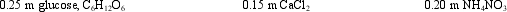
A) C6H12O6 > NH4NO3 > CaCl2
B) C6H12O6 > CaCl2 > NH4NO3
C) CaCl2 > C6H12O6 > NH4NO3
D) CaCl2 > NH4NO3 > C6H12O6
E) NH4NO3 > C6H12O6 > CaCl2
Correct Answer:
Verified
Q43: Explain what is meant by
a. osmosis.
b. osmotic
Q44: Osmotic pressure is the
A) pressure that must
Q45: A coolant consisting of a 50/50 mixture
Q46: Given that the value of kf
Q47: Drinkable water can be produced from seawater
Q49: The driving force for osmosis is
A) temperature.
B)
Q50: In living systems, a solution with a
Q51: What is the vapor pressure of
Q52: A 0.1 M solution of Na2SO4 contains
A)
Q53: Consider these three primary alcohols:

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents